- Healthcare
- Phan Chau Trinh University Hospital
- Phan Chau Trinh University Polyclinic
- TAM TRI QUANG NAM GENERAL HOSPITAL
- Tam Tri Hong Ngu International Hospital
- Tam Tri Cao Lanh General Hospital
- Tam Tri Đong Thap General Hospital
- Tam Tri Nha Trang General Hospital
- Tam Tri Sai Gon General Hospital
- Tam Tri Đa Nang General Hospital
Contact Admission
Device pain relief
Pain relief equipment
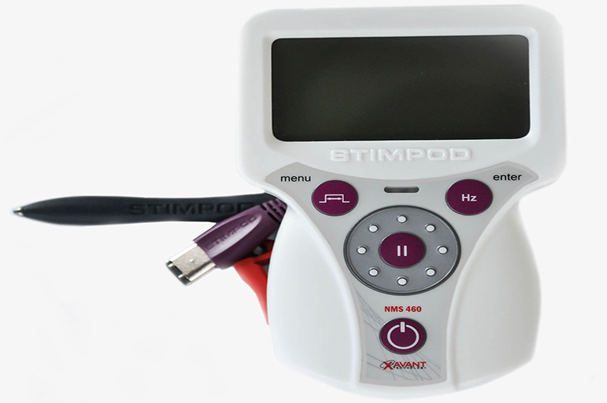
Xavant Technology Company, based in Pretoria, South Africa, has been approved by the FDA (Food and Drug Administration - US Food and Drug Administration) through the peripheral nerve stimulation system NMS 460. This device Used to address chronic pain, postoperative pain, acute post-traumatic pain, and pain control resulting from routine rehabilitation techniques.
This device delivers pulsed frequency waves (PRF - Pulsed Radio Frequency), through the skin. The company claims that the electromagnetic effects are similar to the devices nerve stimulating produces, offering some benefit of the device without natural invasions.
The device also has a nerve fiber distribution probe that helps detect damaged nerves and assesses their status and how they respond to treatment.
.
Source :
Translation: Dr. Nguyễn Hữu Tùng & partner
Other healthcare
- TAM TRI QUANG NAM GENERAL HOSPITAL ( 10:24 - 11/07/2023 )
- Tam Tri Cao Lanh General Hospital ( 15:08 - 20/10/2022 )
- Tam Tri Hong Ngu General Hospital ( 15:05 - 20/10/2022 )
- Phan Chau Trinh University Hospital ( 14:04 - 29/04/2022 )
- Phan Chau Trinh University Polyclinic ( 13:56 - 29/04/2022 )
- Tam Tri Dong Thap General Hospital ( 11:03 - 29/04/2022 )
- Tam Tri Nha Trang Genaral Hospital ( 10:55 - 29/04/2022 )
- Tam Tri Sai Gon Genaral Hospital ( 10:38 - 29/04/2022 )
- TAM TRI DA NANG -- PROUD TO BE YOUR FAVORITE HEALTHCARE PROVIDER ( 10:31 - 29/04/2022 )
- Development history of world medicine ( 11:44 - 13/10/2017 )